Company News
Cancer-fighting immune cells could soon be engineered inside our bodies
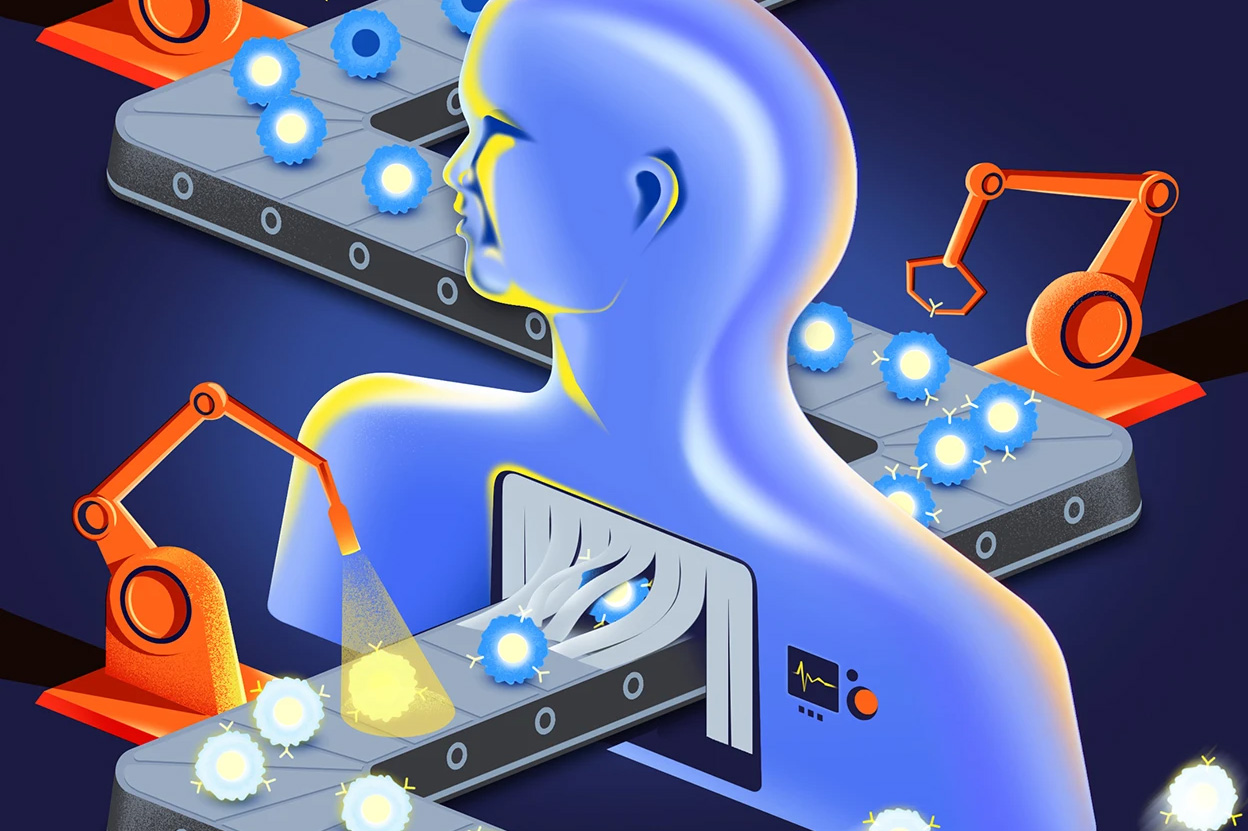
CAR T cells are among the most powerful therapies oncologists have to treat many types of blood cancer. And studies suggest that they might hold promise for brain cancer and other solid tumours, as well as autoimmune and other diseases. One research firm estimates that the value of the CAR-T-therapy market, expected to hit US$11 billion this year, will grow to nearly $190 billion by 2034.
But CAR-T therapies come with a serious downside — they are laborious to make and difficult to administer. Some biotechnology companies have an answer: alter T cells inside the body instead...CRISPR–Cas9 pioneer and Nobel prizewinner Jennifer Doudna has co-founded a separate company, Azalea Therapeutics in Berkeley, California, that is developing in vivo CAR T.