NIH Awards ResVita Bio a $2M Phase II SBIR Grant for Continuous Protein Therapy for Netherton Syndrome
From the press release.
ResVita Bio, a synthetic biology company, announced that the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) at the NIH has awarded the company a $2.03M Phase II Small Business Innovation Research (SBIR) grant to advance RVB-003, a novel treatment for Netherton Syndrome, a chronic and life-threatening genetic skin disorder. This milestone reflects the potential of continuous protein therapy—a groundbreaking new therapeutic paradigm that enables sustained drug production directly on the skin, offering potentially unparalleled safety and efficacy for skin diseases.
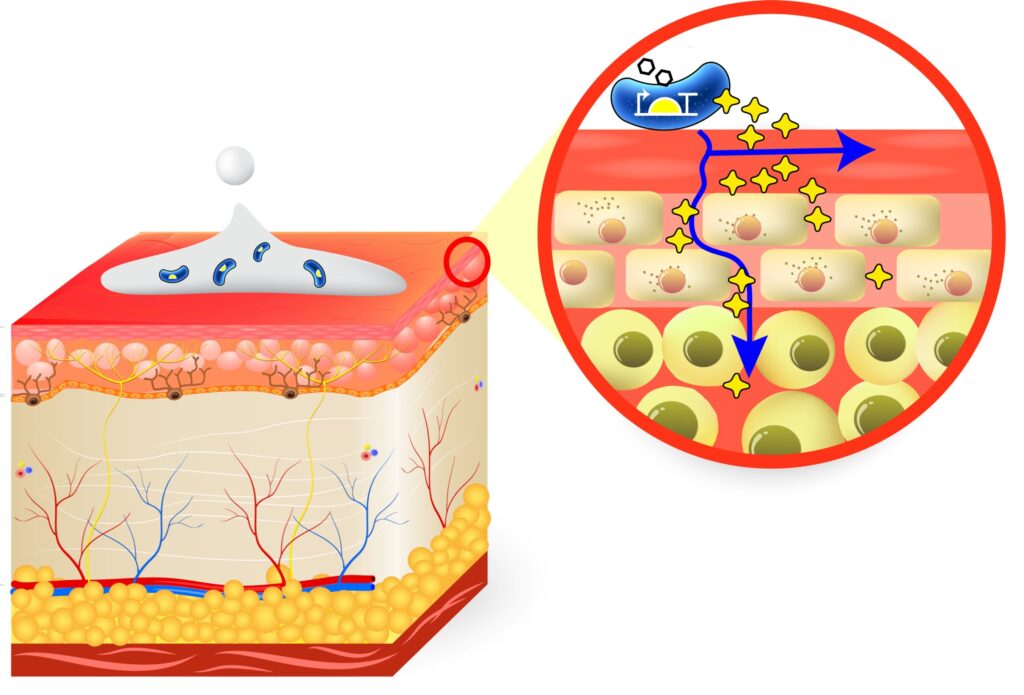
Netherton Syndrome is a rare skin disorder characterized by chronic inflammation, intense itching, and immune manifestations. Infections and dehydration are common due to compromised skin integrity, contributing to high post-natal mortality rates. This genetic disease is caused by disruptions in the SPINK5 gene, leading to an absence of LEKTI, a serine protease inhibitor, which results in uncontrolled protease activity and premature loss of the outermost skin layers.
ResVita Bio is pioneering continuous protein therapy, delivered through a genetically engineered probiotic platform that temporarily colonizes the skin and continuously releases therapeutic proteins, such as LEKTI, to inhibit protease activity. This continuous delivery method ensures high levels of the therapeutic agent at the site of damage, restoring the integrity of the skin barrier. This represents a significant shift from traditional therapies, which may not provide sustained treatment at the site of the disease.
“We are committed to transforming how chronic skin diseases like Netherton Syndrome are treated. Our technology aims to provide continuous, on-site protein therapy, offering a long-lasting solution for patients who currently have few treatment options,” said Dr. Amin Zargar, CEO of ResVita Bio. “The NIH’s support of both our Phase I and Phase II grants is a strong validation of our innovative approach to treating inflammatory skin conditions. With the support of the NIH and our investors, we’ve gone from a back-of-the-envelope idea when we launched in 2022 to the cusp of the clinic, bringing us one step closer to get this much-needed therapy to patients.”
For more information on ResVita Bio and its innovative treatments, visit www.resvitabio.com.
Media Contact: Amin Zargar, 510-328-6703