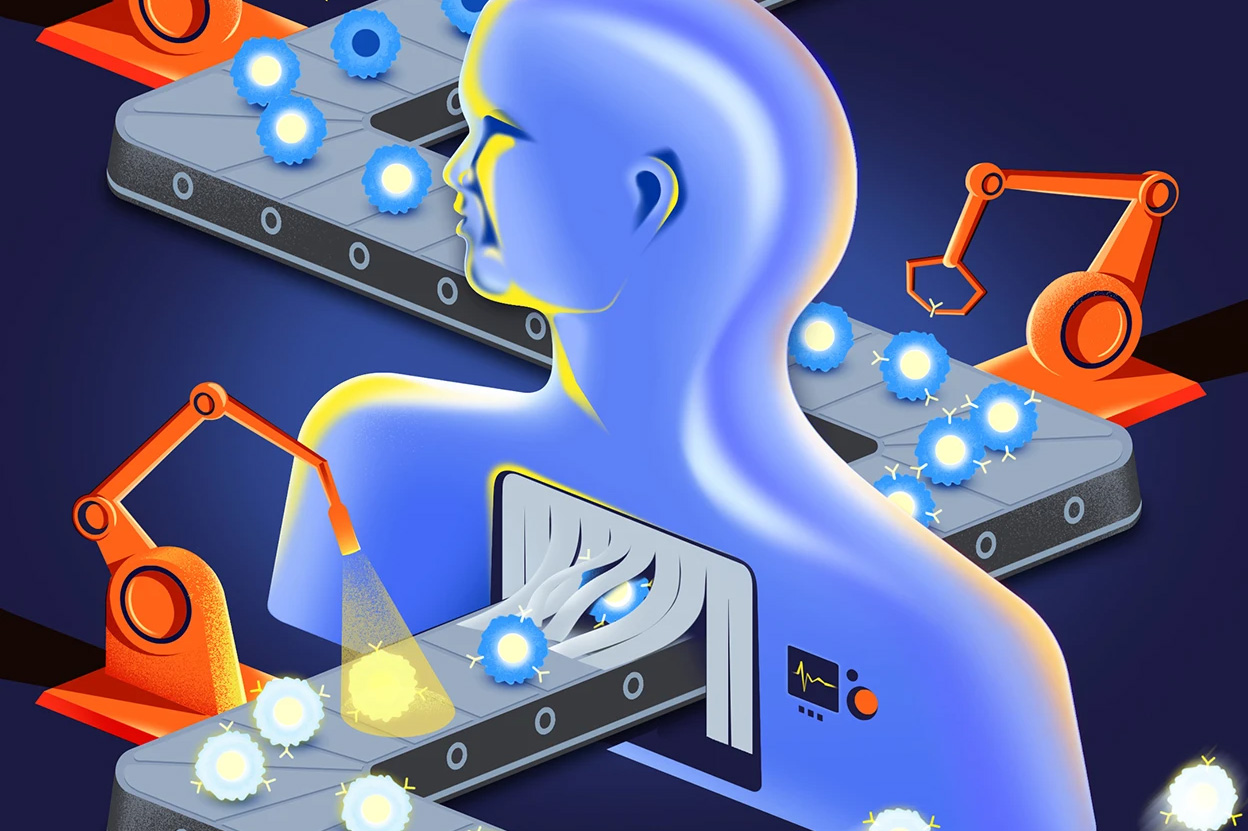
Keylika Develops World’s First Buccal Patch for Iron Deficiency Anemia
From the story in Entrepreneur Asia Pacific.
You probably know at least one person suffering from iron deficiency; however, it still remains one of the top underestimated health problems today. Despite affecting over 10 million Americans and a staggering 1.3 billion people worldwide, treatment options remain stuck in the past – until now. Iron deficiency anemia (IDA) remains a significant public health concern across the Asia-Pacific region, with prevalence rates varying considerably due to factors like socioeconomic status, dietary habits, and healthcare access. South and Southeast Asia, in particular, experience a combined prevalence rate of greater than 52 per cent amongst women of reproductive age.
“Most people have no idea how debilitating iron deficiency anemia can be. As a critical micronutrient, iron has a foundational role to play in multiple biological processes in the body down to the cellular level, its uncorrected deficiency has far-reaching consequences,” says Buddha Chaudhuri, Ph.D., CEO of Biotech company, Keylika. After years working on drug delivery and medical devices, Keylika’s team has pioneered what could be a breakthrough solution: the world’s first buccal (oral) patch for treating iron deficiency.
Bringing Scientific Expertise to the Challenge
With a Ph.D. in drug delivery systems from KU Leuven and Imec in Belgium, Buddha brings extensive expertise to combat iron deficiency treatment. His career spans postdoctoral research at UCSF and UC Berkeley, along with leadership roles directing microsystems technology at medtech startup Biolinq, where he developed wearable continuous glucose monitoring devices.
“My background in microfabrication, materials science and drug delivery systems, together with the personal lived experience of iron deficiency myself, gave me unique insights into how we might solve this widespread problem,” Buddha explains. This specialized knowledge led him to establish Keylika, a Y Combinator-backed Biotech startup now developing a first-of-its-kind solution for iron deficiency.
Symptoms and Challenges in Diagnosis of IDA
Iron is a core component of hemoglobin, the oxygen transporting protein in the body. Without it, oxygen carrying is impacted and virtually every organ system of the body can suffer damage. Sustained iron deficiency can cause persistent fatigue, weakness, shortness of breath, heart palpitations, compromised immunity, musculoskeletal problems, skin and hair issues and even neurocognitive impairment. These symptoms are often debilitating and mask the underlying condition, which is why many doctors dismiss them as stress, anxiety, or depression, particularly in women. This leads to underdiagnosis and undertreatment, perpetuating the cycle of suffering.
Overcoming the Limitations of Existing Treatments
Anyone who has taken iron pills knows the unpleasant side effects, especially at higher doses. Stomach pain, nausea, constipation, diarrhea – it’s no wonder compliance rates are so poor. Dr. Christy Evans, a board-certified Obstetrician & Gynecologist and functional women’s health practitioner at Almond ObGyn in Los Angeles, sees the problem firsthand. “Iron deficiency impacts roughly 40 per cent of adolescent women and up to 35 per cent of adult women of reproductive age,” she explains. “Standard oral supplements have low bioavailability and poor gastrointestinal tolerance, which becomes especially more pronounced in pregnancy.”
Several factors increase the risk for iron deficiency anemia, especially in women, including:
Monthly menstrual blood loss, particularly for women with heavy periods
Pregnancy and post-partum, when iron demands often exceed dietary intake
Underlying conditions that result in iron malabsorption, e.g., inflammatory bowel disease, chronic kidney disease, post-bariatric surgery, cancer, etc.
Vegetarian or vegan diets, as plant sources provide less bioavailable iron, and polyphenols inhibit iron absorption
Intense physical activity, which places a higher burden on oxygen transport, thereby consuming more iron, leading to faster iron depletion
Despite affecting 1 in 4 women globally, iron deficiency often gets misdiagnosed as anxiety, depression, or simply being “tired.” This dismissal leads to misdiagnosis and prolonged suffering for millions.
The alternative isn’t much better. Intravenous (IV) iron infusions work well but require clinical settings, carry risks, and cost a small fortune. According to another physician, Dr. Rondeep Brar, Clinical
Professor of Medicine-Hematology at Stanford School of Medicine: “Such infusions are safe and effective, though require clinical supervision in an infusion center, are not readily accessible to many patients, and carry significant cost.”
Introducing the Buccal Patch: A World First for Iron Delivery
Keylika’s innovative approach centers on a proprietary non-heme iron complex delivered through a dissolvable buccal patch technology called KEYPaxtek™. The patch adheres to the inner oral cheek for less than 30 minutes, allowing iron to absorb directly into the bloodstream, bypassing the gastrointestinal tract and the first pass metabolism. “We’ve developed a new small molecule drug that permeates the buccal mucosa and eliminates the GI side effects associated with oral supplements,” Buddha notes. “Our preclinical studies have demonstrated both safety and efficacy in the buccal delivery of iron into systemic circulation.”
The idea has caught the attention of specialists like Dr. Rondeep Brar, at Stanford. “A novel transbuccal solution could be a valuable addition to available options if a therapeutically relevant dose can be delivered without significant side effects,” notes Dr. Brar. Early tests look promising. In experiments with iron-deficient hamsters, the patch successfully delivered iron directly into the bloodstream without toxic effects. Keylika is exploring potential partnership opportunities with pharma companies to develop the technology further. Dr Evans, who sees many female patients with iron deficiency, can imagine its impact on her patient population, “Keylika’s unique approach of delivering a more absorbable form of iron with a buccal patch can be a game-changer as it has the potential to achieve significantly higher efficacy minus the typical GI side effects of orals.”
Vision for a Platform Technology
Buddha isn’t working alone. His co-founder and CTO, Frederik Ceyssens, Ph.D., brings complementary medical expertise with over 150 published research articles garnering over 2000 citations. Together with advisors from major medical institutions, they are tackling challenges like ensuring the patch is shelf-stable and tastes acceptable while scaling up manufacturing. “While iron deficiency constitutes a huge unmet medical need, and is our first go-to clinical indication, this is not our only asset,” says Buddha.
Frederik Ceyssens explains, “Our buccal patch has been demonstrated as a platform drug delivery technology, one that can be used to deliver a wide range of molecules less than 10 kDa molecular size that suffer from poor oral bioavailability, directly into blood circulation without the need for injections.” In that, Keylika’s buccal technology represents a step-change in oral drug delivery norms, with the overarching goal of administering hard-to-deliver drugs systemically, bridging molecular size and dosage constraints.
The company’s next strategic asset class is peptides and hormones, with the KEYPaxtek™ delivery tech exhibiting the potential to deliver these molecules. Keylika was recently accepted into Bakar Labs at UC Berkeley, giving them access to additional resources and expertise.
The next major milestone will be more preclinical tests culminating in first-in-human studies, followed by FDA submissions. “Our goal has always been to find solutions that actually work for patients,” Buddha says. “When you’re iron deficient, you just want to feel better without trading in one problem for another.” If Keylika succeeds, millions of people might finally have an option that doesn’t force them to choose between adverse oral side effects or hasslesome injections.