Uncategorized
ResVita Bio Announces RVB-003 granted Orphan Drug Designation for Netherton Syndrome by the FDA
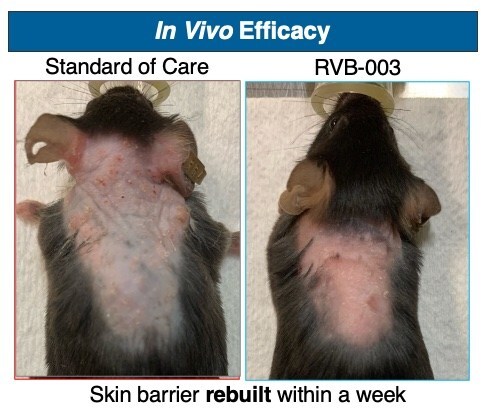
ResVita Bio, a therapeutics company focused on treatments for skin diseases, today announced that the FDA awarded the Orphan Drug Designation to RVB-003 for the treatment of Netherton Syndrome, a chronic and life-threatening skin disorder. This milestone, following the FDAs previous granting of the Rare Pediatric Disease Designation, underscores the impact of ResVita Bio's new platform for continuous protein therapy—a groundbreaking therapeutic approach designed to deliver sustained drug levels directly to the skin, supporting both greater efficacy and improved safety over other topicals.